

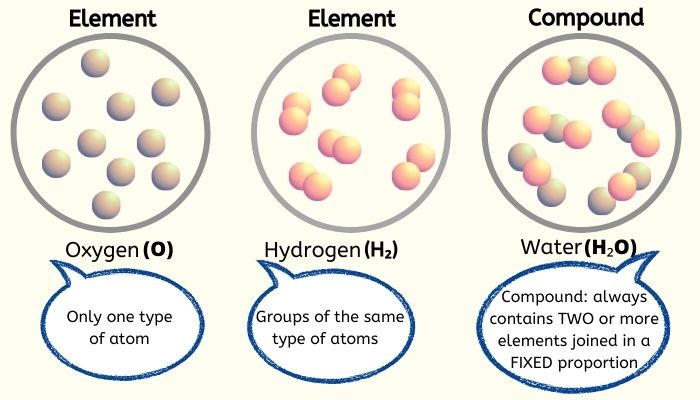
John Dalton at times was known as the father of modern atomic theory. When was it believed that atoms of a given element are identical? Most elements as they occur naturally on earth are mixtures of several isotopes. REMEMBER: Atoms of the same chemical element do not always have the same mass because, although the number of protons in the nucleus is the same for all atoms of the same element, the number of neutrons is not. (2) All atoms of the same element are identical different elements have different types of atom. It involves the following postulates: (1) Elements consist of indivisible small particles (atoms). Who said all atoms of a given element are identical in mass and properties?Ī theory of chemical combination, first stated by John Dalton in 1803. Since the states of the electrons in an atom are what determine the nature of the chemical bonding that the atom experiences, two atoms of the same element can react differently if they are in different states. Two atoms of the same chemical element are typically not identical. Is it true that atoms of the same element are identical? 6 How is the atomic mass different from the mass number for an element?.5 Do all atoms of an element have the same mass?.
4 When was it believed that atoms of a given element are identical?.3 Who said atoms of the same element are identical and are different from atoms of other elements?.2 Who said all atoms of a given element are identical in mass and properties?.1 Is it true that atoms of the same element are identical?.


 0 kommentar(er)
0 kommentar(er)
